An absolute resistance to corrosion cannot be achieved technically. Therefore all of our products are equipped with a zinc alloy. This serves as means of protection in order to prevent deterioration of the device during its serviceable
life.
Due to aggressive influences of environment the zinc alloy wears off with time though. The speed in which this can occur can be influenced by various methods of zinc-coating.
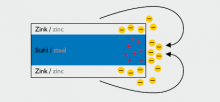
Active cathodic protection against corrosion
Since zinc is less noble than iron in the electromechanical contact displacement series, the coating functions as anode and protects the less noble material - iron - in cathodic form until the zinc has been corroded off.
You can find the following versions in our catalogues:
- Sendzimir zinc-coating (band zinc-coating) acc. DIN EN 10327 (formerly DIN EN 10147 and DIN 10142) middle value lamination strength app. 20 μm.
Interfaces are secured by means of the cathodic protection against corrosion up to a material thickness of 2 mm. - Hot-dip zinc-coated (piece zinc-coating) acc. DIN EN ISO 1461 middle value lamination strength app. 40-60 μm.
Interfaces must be zinc-coated as aftertreatment. - Galvanically zinc-coated (electrolytic zinc-coating acc. DIN EN 12329) Middle value lamination strength app. 2,5 - 10 μm.
- V2A stainless steel material number 1.4301 or American Norm 304
| Class | Inside | Outside | Corrosion Aggressiveness | Average Decomposition of Zinc Thickness / Year |
| C1 | bheated rooms for example offices | - | inconsequential | < 0,1 μm/year |
| C2 | unheated rooms for example warehouses | rural areas | little | 0,1 bis 0,6 μm/year |
| C3 | high humidity little to medium air pollution for example creameries, breweries | municipal and industrial areas with moderate pollution |
moderate | 0,6 bis 2,3 μm/year |
| C4 | chemical plants, swimming pools | municipal and industrial areas with high pollution |
strong | 2,3 bis 4,5 μm/year |
| C5 | rooms with strong and continuous condensation | shore and offshore areas | very strong | >4,5 bis 8,4 μm/year |